Endoscopic Ultrasound in Gastrointestinal Endoscopy: Breaking through barriers
By Todd H. Baron, MD, Division of Gastroenterology and Hepatology, University of North Carolina in Chapel Hill, Chapel Hill, North Carolina
Traditional gastrointestinal endoscopic procedures are performed using digital camera technology to examine the lining of the esophagus, stomach, small bowel and colon. Diagnostic endoscopy using these endoscopes has evolved over the years to allow numerous therapeutic interventions within the gastrointestinal lumen. In 1980, the first description of endoscopic ultrasound was reported from a pancreatic specialist at Mayo Clinic, Rochester. The initial images were suboptimal, and the report was written with a skeptical undertone as to the future of the technology. Endoscopic ultrasound (EUS) is achieved by the attachment of an ultrasound probe to the tip of a flexible endoscope. It allows visualization of surrounding structures when positioned internally within the gastrointestinal lumen. These endoscopes are most often passed orally with the patient sedated, to assess upper gastrointestinal pathology. They can also be passed transrectally for assessment of pathology in and around the rectum and colon. Since ultrasound waves fall off rapidly with distance from the probe with higher frequency (which provides better resolution), one can overcome the distance factor since the proximity of the probe to surrounding structures can be in the millimeter range, with resolution of two structures at higher frequency down to a single millimeter. This cannot be achieved with any other imaging modality. EUS was Initially developed as a diagnostic “look but can’t touch” for cancer staging, the pancreas assessment, and the evaluation of underlying bile duct stones. However, over time, these initial radial echoendoscopes gave way to linear echoendoscopes. Linear endoscopes allow not only visualization of surrounding structures but, in contrast to radial ultrasound probes, instruments that are passed through the endoscope channel can be seen in real-time as they pass the ultrasound probe. The first application of linear EUS was needle sampling of tumors to obtain a tissue diagnosis to allow the administration of oncologic therapy. Pancreatic carcinoma is now definitively diagnosed using EUS. It is the gold standard for obtaining needle biopsies, as the entire pancreas can be seen when the endoscope is positioned in the stomach and duodenum, again due to its close proximity to surrounding structures. EUS-guided pancreatic biopsies in patients with pancreatic tumors seen by CT or MRI This has replaced older open surgical biopsies and CT-guided biopsies, the latter of which can cause tumor seeding along the pathway and a potential source of recurrent cancer after otherwise definitive surgical resection. Similarly, lymph nodes, liver, and almost any adjacent structure can be needle-biopsy sampled, as long as the endoscope can reach the target.
EUS-guided gastrointestinal therapeutics have opened the door to a new world of therapeutic endoscopy. Future developments are likely to include newer stent designs and drug delivery into tumors.
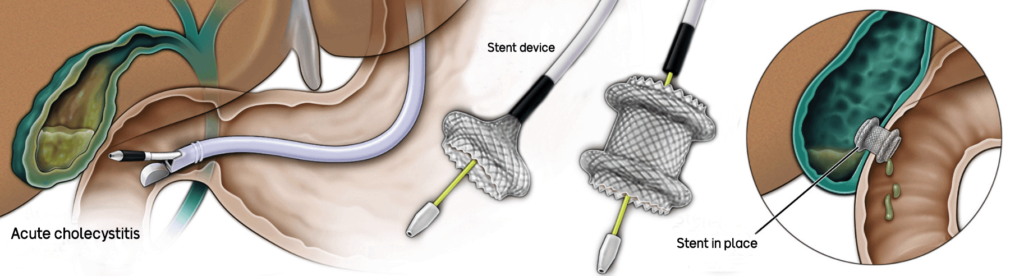
Therapeutic applications soon followed, though the more invasive procedures are currently performed at tertiary/specialized centers. For example, a colleague and I first described draining an infected gallbladder through the duodenum (first portion of the small intestine) in 2007. This permitted non-surgical management, and in this patient’s cancer condition, the only other viable option was to have one of our interventional radiology colleagues place a drain into the gallbladder from the outside, which would have remained in place for life and negatively impact the quality of life. Thus, internal endoscopic drainage procedures have emerged to supplant surgery and interventional radiology in selected patients with diseases of the bile duct and pancreatic duct. Newer designed self-expandable metal stents have been developed to bring two luminal structures that are not normally apposed (adhered) to one other. Such a device, termed a luminal-apposing metal stent (LAMS), is now FDA-approved for gallbladder drainage in poor operative candidates. It has an electrocautery-enhanced tip that allows the device to be passed across the gastrointestinal wall into the adjacent structure (e.g., gallbladder) under EUS-guidance. Doppler capability on the ultrasound probe allows identification and avoidance of intervening blood vessels.
Another example of therapeutic EUS is the ability to create an endoscopic bypass of a blockage in the stomach or small intestine (again, most commonly in the setting of advanced pancreatic cancer), termed endoscopic gastroenterostomy, to allow the patient to eat normally. In centers with expertise in performing this procedure, it has replaced surgical bypass with at least a good functional outcome, shorter hospitalization, and a more rapid return to oral intake. It can often be performed in the outpatient setting as a same-day procedure with discharge home from the recovery unit.
Another example of the therapeutic application of EUS is the ability to intervene on bleeding vascular lesions within the gastrointestinal tract (most commonly gastric [stomach] varices) using EUS-directed needle puncture into the vessel and delivering metal coils and glue to thrombose (clot) the vessel.
Radiofrequency ablation probes passed through needles have been developed to allow for EUS-guided tumor destruction. Small pancreatic neuroendocrine tumors can be destroyed, and small case series have shown oncologic results similar to surgical resection without the accompanying morbidity.
In summary, EUS-guided gastrointestinal therapeutics have opened the door to a new world of therapeutic endoscopy. Future developments are likely to include newer stent designs and drug delivery into tumors.

